What Does the Law of Constant Composition Mean
Learn vocabulary terms and more with flashcards games and other study tools. Law of definite proportions - law stating that every pure substance always contains the same elements combined in the same proportions by weight law.
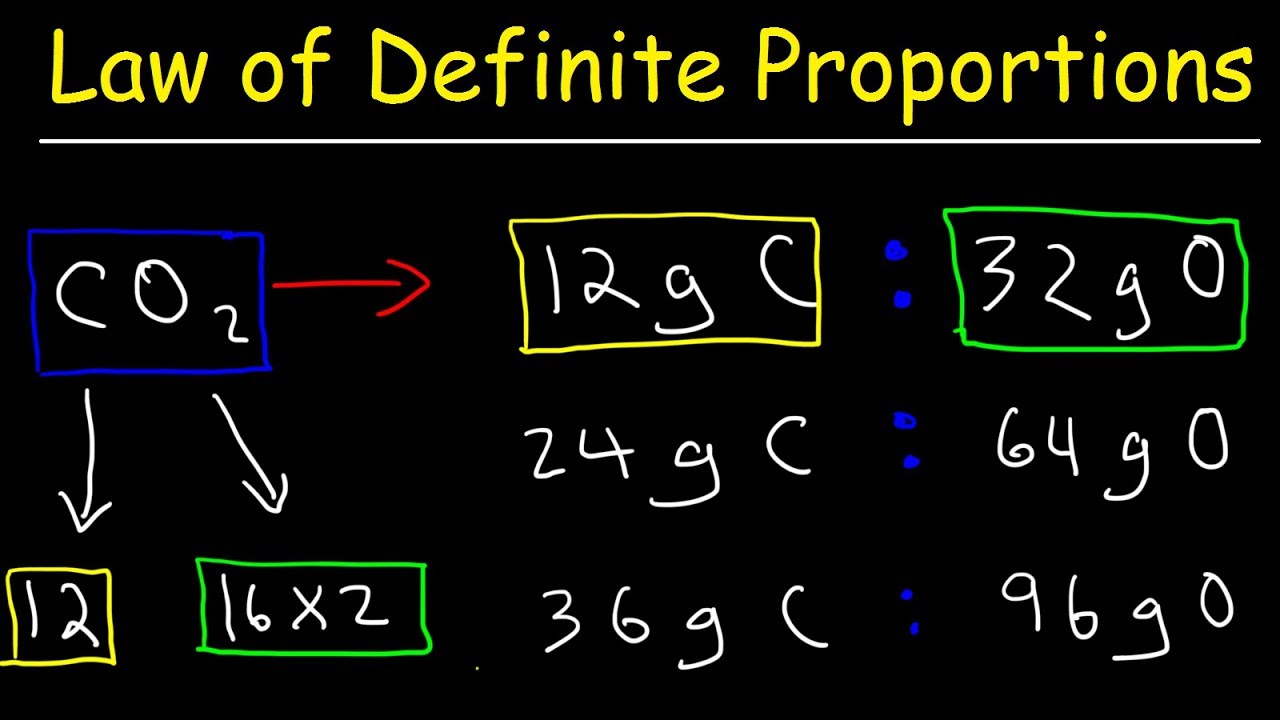
Law Of Definite Proportions Chemistry Practice Problems Chemical Fundamental Laws Youtube
Condensation of water vapor.

. Law of definite compositionA law that states that chemical compounds are formed of constant and defined ratios of elements as determined by mass. This implies that any pure sample of a compound no matter the source will always consist of the same elements that are present in the same ratio by mass. The law of constant composition says _____.
In the following list only _____ is NOT an example of a chemical reaction. If you want to mix one spoonful of sugar with your tea you can. Two or more elements when united to form a new substance do so in a constant and fixed proportion by weight.
In chemistry the law of definite proportion sometimes called Prousts law or law of constant composition states that a given chemical compound always contains its component elements in fixed ratio by mass and does not depend on its source and method of preparation. The Law of Definite Proportions sometimes called Prousts Law states that any chemical compound always contains the same elements in the same proportion by mass. The law of constant composition states that the chemical compounds consist of the elements which are present in a fixed ratio by their mass.
Does the ratio of the mass of reacted R or Nu to the mass of product formed depend on the mass of the materials you were given to work with. Learn more about the definition. The law of constant composition says that in any particular chemical compound all samples of that compound will be made up of the same elements in the same proportion or ratio.
For example pure water will always contain hydrogen and oxygen in a fixed mass ratio a gram. Chemistry is the study of the change of matter from one form to the other. 1 Answer Ernest Z.
The law of constant proportions states that chemical compounds are made up of elements that are present in a fixed ratio by mass. If you want to mix two you. That the composition of a compound is always the same.
These changes often occur as a result of the combination of two different types of matter. Does this model agree with the Law of Constant Proportions. This means that any given pure sample of the compound irrespective of its source will always contain the same kind of elements that are present in the same ratio by the mass.
ElementAny one of the simplest chemical substances that cannot be decomposed in a chemical reaction or by any chemical means and are made up of atoms all having the same number of protons. The law of definite proportion sometimes called Prousts law or law of constant composition states that a given chemical compound always contains its component elements in fixed ratio by mass and does not depend on its source and method of preparation. The law of constant composition states that when elements are chemically combined they are always present in certain proportions by weight.
The law of constant composition says that in any particular chemical compound all samples of that compound will be made up of the same elements in the same proportion or ratio. Learn the basics about the law of constant composition and how to apply itThe law of constant composition states that in a given chemical compound all samp. For example any water molecule is always made up of two hydrogen atoms and one oxygen atom in a.
Law of Constant Composition or Definite Proportions. The law of constant proportions states that chemical compounds are made up of elements that are present in a fixed ratio in terms of their mass. In chemistry the law of constant composition also known as the law of definite proportions states that samples of a pure compound always contain the same elements in the same mass proportion.
The Law of Constant Composition discovered by Joseph Proust is also known as the Law of. Law of constant composition - definition of Law of constant composition by The Free Dictionary. Would the ratio have been the same if the rings or bolts were heavier.
Laws of Chemical Combinations. This specifies that any pure sample of a compound no matter what is the source of that compound will always encompass the same elements that are present in a similar ratio by their mass. What do we mean by this.
3 What is the law of constant composition with example. Start studying Law of Constant Composition. Jan 8 2014 A particular compound will always contain same elements in same constant proportion irrespective of its source or method of preparation Nowadays Prousts Law of Constant Composition is usually known simply as the Law of.
Dissolution of a penny in a nitric acid condensation of water vapor burning candle formation of polyethylene from ethylene or rusting of iron. Law of Definite Proportions Law of Constant Composition - A given compound always has the same elements in the same proportion by mass. For example any water molecule is always made up of two hydrogen atoms and one oxygen atom in a 21 ratio Advertisement Still have questions.
This is known as the law of constant composition. Laws of constant proportion It states that in a chemical substance the elements are always present in a fixed proportion by their mass. French chemist JL.
This law together with the law of multiple proportions is the basis for stoichiometry in chemistry. There is no definite formula for the composition of a mixture. 4 When 320 grams g of methane are burned in 1280 g of oxygen 880 g of carbon dioxide and 720 g of water are produced which law is this an example of.
Let us read about it below.

Law Of Definite Proportions Law Of Constant Composition

Law Of Constant Proportions Don T Memorise Youtube

Law Of Constant Composition Properties Of Matter Chemistry Fuseschool Youtube

Difference Between Law Of Constant Composition And Law Of Multiple Proportions Compare The Difference Between Similar Terms
Laws Of Chemical Combination Overview Explanation Video Lesson Transcript Study Com

Law Of Constant Proportions Statement Explanation Exceptions Faqs

How To Write A Chemical Reaction In Chemical Equation Form Chemical Equation Chemical Reactions Chemical

Law Of Definite Proportions Law Of Constant Composition
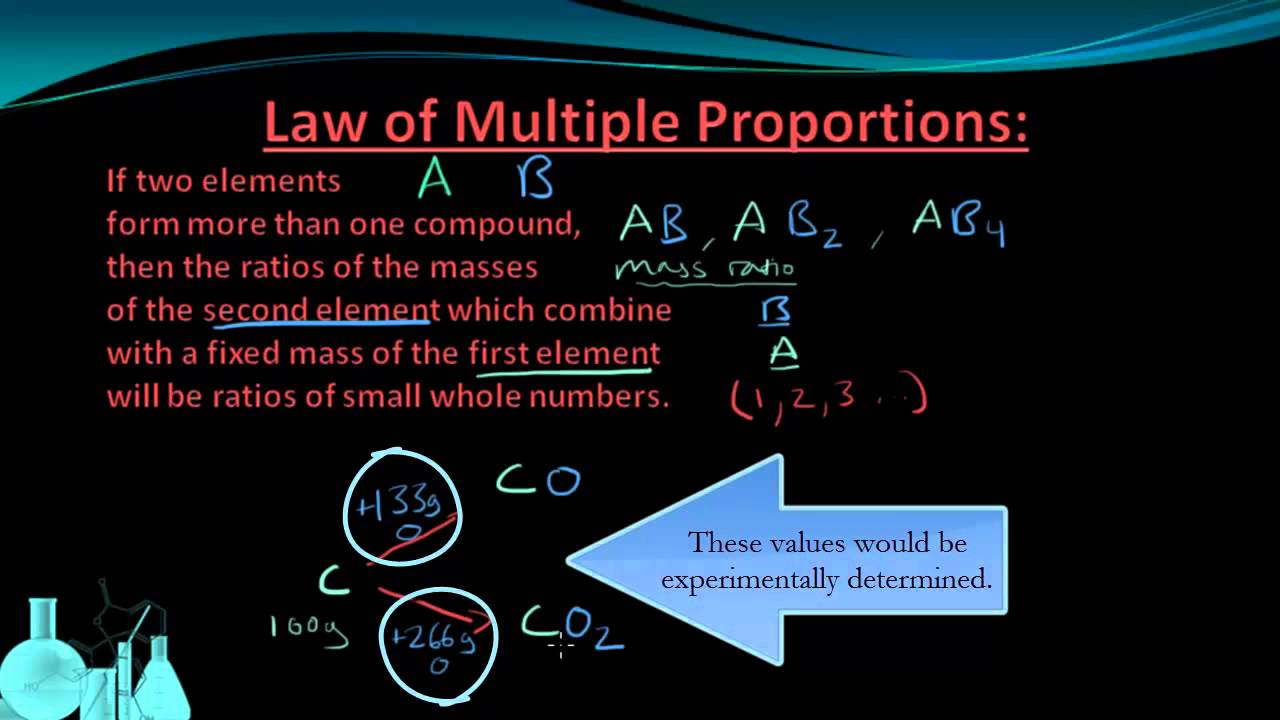
Early Ideas In Atomic Theory Chemistry For Majors

Difference Between Law Of Constant Composition And Law Of Multiple Proportions Compare The Difference Between Similar Terms

Law Of Definite Proportions Law Of Constant Composition

Difference Between Law Of Constant Composition And Law Of Multiple Proportions Compare The Difference Between Similar Terms

Difference Between Law Of Constant Composition And Law Of Multiple Proportions Compare The Difference Between Similar Terms

Law Of Definite Proportions Chemistry Practice Problems Chemical Fundamental Laws Youtube

Combination Reaction Reactions Combination Chemistry
4 3 Dalton S Atomic Theory Chemistrysaanguyen

Law Of Definite Proportions Chemistry Practice Problems Chemical Fundamental Laws Youtube

Heterogeneous Equilibrium Easy Science Equilibrium Easy Science Chemistry Notes

Comments
Post a Comment